Introduction to Pharmacovigilance: Ensuring Safe and Effective Healthcare
Pharmacovigilance, a crucial aspect of healthcare, plays a vital role in ensuring the safety and efficacy of medications. As the pharmaceutical industry continues to evolve, the importance of pharmacovigilance cannot be overstated. In this blog, we’ll delve into the world of pharmacovigilance, exploring its significance, key concepts, and career opportunities.
What is pharmacovigilance?
Pharmacovigilance is the science and activities related to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problems. This includes monitoring and managing risks associated with medications, vaccines, and medical devices.
Why Is Pharmacovigilance Important?
Pharmacovigilance is essential for:
- Patient Safety: Identifying and mitigating medication risks to protect patients.
- Public Health: Preventing adverse events and promoting safe medication use.
- Regulatory Compliance: Ensuring adherence to international standards and guidelines.
- Medical Advancements: Informing drug development and improving treatment outcomes.
Key Concepts in Pharmacovigilance –
- Adverse Event Reporting: Collecting and analysing data on medication-related adverse events.
- Signal Detection: Identifying potential safety issues through data analysis.
- Risk Management: Developing strategies to minimise medication risks.
- Pharmacovigilance Regulations: Compliance with global regulations & guidelines.
Career Opportunities in Pharmacovigilance
Pharmacovigilance offers a range of career paths for entry-level professionals.
- Pharmacovigilance Officer: Monitoring and reporting adverse events.
- Drug Safety Specialist: Analysing and mitigating medication risks.
- Regulatory Affairs Specialist: Ensuring compliance with regulations.
- Clinical Research Associate: Conducting pharmacovigilance studies.
Skills Required for a Career in Pharmacovigilance
- Analytical and problem-solving skills.
- Knowledge of regulations and guidelines.
- Communication and collaboration skills.
- Data management and analysis skills
Why Should You Opt for Pharmacovigilance as Your Career?
- 💊 Key Reasons to Choose Pharmacovigilance
Pharmacovigilance is the science and activities relating to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problems.
📈 Strong Career Growth and Demand
The demand for trained PV professionals is increasing globally due to tightening regulatory requirements and the continuous launch of new drugs.
- Growing Industry: As the pharmaceutical and biotechnology industries expand, so does the need for experts to monitor the safety profile of their products.
- Diverse Roles: Opportunities exist for both freshers and experienced professionals in roles like Drug Safety Associate (DSA), Pharmacovigilance Scientist, Medical Reviewer, and PV Manager.
- Attractive Salaries: The field offers competitive salaries, which tend to increase significantly with experience and specialisation (e.g., in signal detection or risk management).
- Technological Integration: The adoption of AI and automation for analysing large datasets is creating new opportunities for tech-savvy professionals in PV.
🧑⚕️ Critical Role in Public Health
PV professionals play a vital part in ensuring patient safety and the ethical use of medicines.
- Protecting Patients: You contribute directly to protecting public health by continuously monitoring and evaluating the safety of medicines after they are available to the public.
- Risk Management: You help identify, assess, and prevent Adverse Drug Reactions (ADRs) and ensure the benefits of a drug continue to outweigh its risks.
- Regulatory Compliance: You ensure pharmaceutical companies comply with stringent global and local regulations (like those from the FDA and EMA) for drug safety reporting.
🚪 Accessibility for Life Science Graduates
Pharmacovigilance is particularly accessible to those with a background in biological sciences.
- Relevant Backgrounds: It’s an excellent field for students of pharmacy, life sciences, biotechnology, and medicine.
- Entry-Level Opportunities: Many freshers start in entry-level roles such as a drug safety associate, focusing on tasks like case processing, data entry, and using dictionaries like MedDRA for coding adverse events.
How Has the Industry Worked Out So Far?
The Pharmacovigilance (PV) industry has worked out very well so far, transforming from a largely manual, reactive function into a rapidly growing, technologically advanced, and critically important sector of the pharmaceutical and healthcare industry.
📈 Current State: Strong Growth and Outsourcing Trend
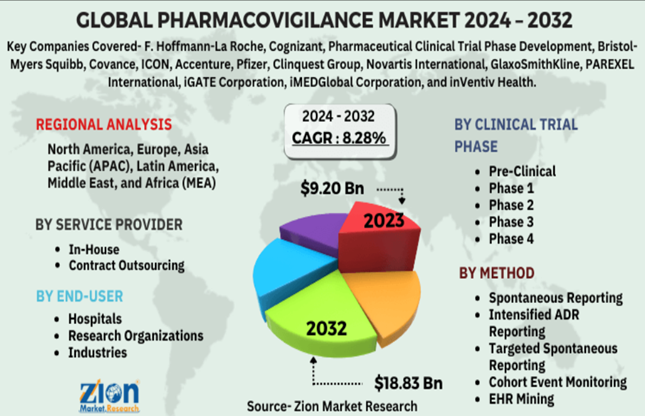
The pharmacovigilance market is experiencing robust growth globally, driven by stringent regulations and the continuous launch of new, complex drugs.
- Market Expansion: The global pharmacovigilance market is valued in the billions of US dollars and is projected to see a high Compound Annual Growth Rate (CAGR) over the next several years.
- Outsourcing Boom: There is a significant and increasing trend of contract outsourcing of PV services to specialised vendors (contract research organisations, or CROs). This allows pharmaceutical companies to manage costs, access global expertise, and ensure compliance without maintaining large in-house teams for routine tasks.
- Post-Marketing Focus: The Phase IV (post-marketing) surveillance segment dominates the market, as regulators and drug manufacturers demand continuous monitoring of products once they are widely available to the public.
💻 Key Trends Driving the Industry’s Evolution
- The industry is undergoing a digital transformation that is fundamentally changing how drug safety is monitored.
| Trend | Description | Impact on PV Work |
|---|---|---|
| Artificial Intelligence (AI) & ML | AI and machine learning are being adopted to automate routine, labour-intensive tasks like case intake. Triage and data coding (MedDRA). | Faster, more accurate processing of adverse event reports enables earlier signal detection. |
| Real-World Evidence (RWE) | Increased integration of data from non-traditional sources like Electronic Health Records (EHRs), claims data, and patient registries. | Provides a broader, more realistic view of a drug’s safety profile outside of controlled clinical trials. |
| Patient-Centric PV | A shift towards empowering patients to directly report adverse events (Patient-Reported Outcomes/Experiences) via mobile apps and other digital platforms. | Enhances data collection and provides real-time insights into patient experiences. |
| Regulatory Harmonization | Global regulatory bodies (FDA, EMA, WHO) are continually working to harmonise standards to facilitate global reporting and compliance. | Streamlines workflows for multinational companies and improves the efficiency of international data sharing. |
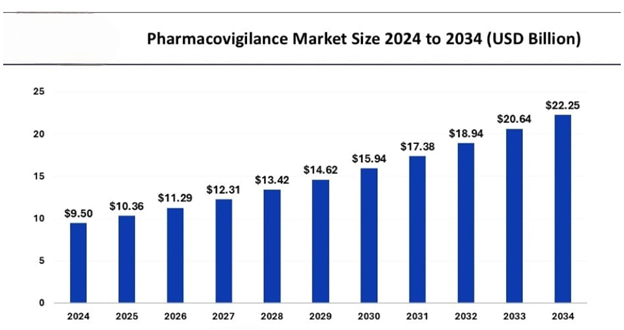
This change has brought about the creation of a huge number of job opportunities related to this sector. Clinical research and pharmacovigilance courses make you a step ahead to stand out and to get hired in this industry. The PhV market is moderately competitive and consists of several major players. Companies like Accenture, IBM Corporation, Wipro, Cognizant, and Merck hold substantial market share in the PhV market. The Asia Pacific market accounted for a significant industry share in 2024 and is poised to exceed USD 22.25 billion by 2034, according to a new report published by Global Market Insights, Inc.
Job and career prospects
The growth in PhV is fast, which eventually leads to managerial and director roles with good pay packages.
The entry-level job for life science graduates/postgraduates is DSA (Drug Safety Associate or Senior Drug Safety.
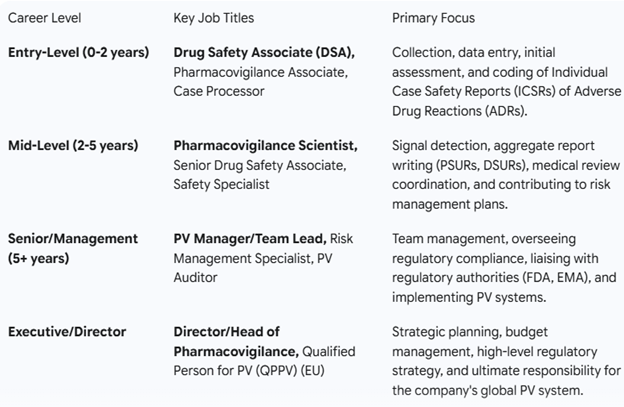
Associate). DSAs are mainly involved in case creation, processing, evaluation, checking for MSI (minimum safety information—a patient, a reporter, a suspect drug, and an adverse event), reconciliation and follow-up process, data entry of all information available in the document and medical coding, and narrative writing.
From there, career progression in PhV will typically grow with exposure, knowledge, and experience. Your role can vary from process expert, subject matter expert, p
roduct quality lead, team leader, assistant manager, deputy manager, senior manager, general manager, director, vice president, service delivery manager/lead, and many more. There is continuous growth in the field with an average 30% raise in salary every year. The yearly compensation for a career in PhV is very good, and the starting salary ranges.
Apart from these, your role can also involve more in-depth knowledge of the technical part if you acquire the additional skills in PhV as below. Salary packages reach high with each role or designation, up to 50-60 lakhs or more per annum based on experience, tenure, knowledge, and skills.
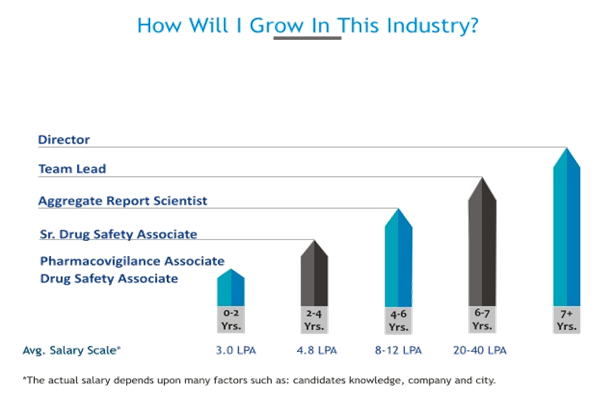
Growth Prospect:
The pharmacovigilance field offers stable, high-demand, and rewarding career prospects for those with a background in life sciences, pharmacy, or medicine. The increasing stringency of global drug safety regulations and the growing number of clinical trials ensure a consistently high need for PV experts.

What Training programs/courses can be taken to become a Pharmacovigilance Professional at Dysmech Clinical Services Pvt Ltd (DCS)
- Post Graduate Diploma in Advanced Clinical Research Management (CRM) (1 year) (9 months theory + practical & 3 months internship)
- Post Graduate Diploma in Clinical Research (CR) and Pharmacovigilance (PV) (1 year) (9 months theory + practical & 3 months internship)
- Post Graduate Diploma in Clinical Research (CR) and Clinical Data Management (CDM) (1 year) (9 months theory + practical & 3 months internship)
- Post Graduate Diploma in Clinical Research (CR) and Regulatory Affairs (RA) (1 year) (9 months theory + practical & 3 months internship)
- Advanced Diploma in Clinical Research Management (CRM) (6 months theory + practical & 1 month internship)
- Diploma in Health Technology Assessment (HTA) (6 months)
- Diploma in Good Clinical Practices (GCP) (6 months)
- Diploma in Advanced Clinical Research (CR) and Pharmacovigilance (PV) (6 months theory + practical & 1 month internship)
- Diploma in Clinical Research (CR) and Clinical Data Management (CDM)
(6 months theory + practical & 1 month internship)
- Diploma in Clinical Research (CR) and Regulatory Affairs (RA) (6 months theory + practical & 1 month internship)
- Certificate course in Clinical Research (CR) (3 Months)
- Certificate course in Pharmacovigilance (PV) (3 Months)
- Certificate course in Clinical Data Management (CDM) (3 Months)
About Dysmech Clinical Services Pvt Ltd (DCS):
DCS is an educational research centre, a professional training and soft skill development organisation. Our company provides the best and state-of-the-art facilities and knowledge-sharing platforms, which are an exception in the PV industry.
DCS offers specialised programs with industry exposure in international standards, with Drug Information Association (DIA) courses in Pune.
Our faculty and guest lecturers are from the industry itself, and they are astute in delivering their duties as and when required by the students. Solving the queries of the students and taking them to the right path is what our faculty is masterful at.
The ambience and study environment at @DCS is one of the best compared to the other institutes providing Pharmacovigilance courses in Pune.
The teaching staff @at DCS help to learn the depth of this subject and course, and helps them get certified, which is internationally acclaimed in collaboration with some of the prestigious partners.s
Drug Information Association, which adds volume to their profile and makes each of our students get a practical exposure to software tools along with theory classes, which help them get trained and become proficient to enter the industry.
Students registered with us @DCS would have an excellent platform and knowledge to land in a good company or enterprise, and can boost their growth opportunities by availing the benefits of our wide network and contacts across companies and industries.
Looking at the growing demand for the courses, DCS has introduced the state-of-the-art curriculum, which will help our students cope with the requirements and pressures of the real job. For the benefit of working professionals and full-time students, the course is conducted every Saturday.
You can very well trust in our honest dealings.
So, when are you joining??
Check out the details and contact us today to be the leader of tomorrow.
