Translating Science into Health : The Core of Clinical Research
Clinical research is the scientific study of health and illness in people to improve human health by discovering new ways to prevent, diagnose, and treat disease. It involves systematic studies on human volunteers and is different from clinical practice because it collects evidence under rigorous conditions to determine the safety and effectiveness of new treatments, devices, and diagnostic products. Key components include clinical trials (which test interventions) and observational studies (which watch how treatments and diseases work).
What is clinical research?
Clinical research is a branch of healthcare science that studies health and illness in people. Its primary goal is to determine the safety (tolerability) and effectiveness (efficacy) of new medical interventions, such as
- Drugs and Vaccines
- Medical Devices
- Surgical Procedures
- Diagnostic Tools
- Treatment Regimens or Behavioral Changes
Why is clinical research important?
Clinical research is essential for:
- Developing New Treatments and Cures
- Ensuring Safety and Efficacy
- Advances in Medical Knowledge
- Improves Public Health and Quality of Life
- Fosters Personalized Medicine
Key Concepts in Clinical Research—
- Randomization: The process of assigning trial participants to different treatment groups (e.g., intervention or control) by chance (like flipping a coin or using a computer program).
- Control Group: The group of participants who receive the standard treatment, a placebo, or no intervention (depending on the study design).
- Placebo: An inactive intervention (e.g., a “sugar pill” or a sham procedure) that looks, tastes, and is administered identically to the active drug or intervention.
- Blinding (Masking): The process of keeping one or more parties in the trial (participants, doctors, data analysts) unaware of which treatment assignment a participant received.
Career Opportunities in Clinical Research
Clinical research offers a range of career paths for entry-level professionals.
- Clinical Research Coordinator (CRC): Manages study activities at the site (e.g., hospital, clinic). Responsibilities include patient recruitment, screening, scheduling visits, data entry, and managing regulatory documents.
- Clinical Research Associate (CRA) / Monitor: Works on behalf of the sponsor (or CRO) to monitor trial sites. They ensure the trial adheres to the protocol, GCP (Good Clinical Practice), and regulatory requirements, and verify the accuracy of the data collected (Source Data Verification).
- Clinical Trial Manager (CTM): Oversees the daily operations of a clinical trial or a set of trials. Manages study budgets, timelines, vendors, and the team of CRAs.
- Director of Clinical Operations: Provides strategic oversight for the entire clinical operations department, focusing on efficiency and quality across multiple trials.
Skills Required for a Career in Clinical Research
- Analytical and problem-solving skills.
- Knowledge of regulations and guidelines.
- Communication and collaboration skills.
- Data management and analysis skills
Why Should You Opt for Clinical Research as Your Career?
💊 Key Reasons to Choose Clinical Research
A career in clinical research is an excellent choice for individuals who want to combine their passion for science and healthcare with st
rong organizational and project management skills. It offers a unique blend of intellectual fulfillment, professional stability, and real-world impact.
🔬 High-Impact and Fulfilling Work
The primary motivation for many in clinical research is the opportunity to be directly involved in advancing medicine.
- Making a Difference: Your work directly contributes to the development and approval of new drugs, medical devices, and therapies. You are a crucial link in the process of bringing life-saving treatments to patients globally.
- Scientific and Medical Learning: The field offers constant intellectual stimulation. You are always learning about cutting-edge medical advancements and new therapeutic areas, as no two studies are the same.
- Ethical Foundation: The core of clinical research is rooted in Good Clinical Practice (GCP) and patient safety, ensuring that all research is conducted ethically and with integrity.
📈 Strong Career Growth and Stability
The industry’s structure and global demand provide numerous opportunities for long-term professional development.
- High Demand and Job Stability: Clinical research is considered a recession-resilient industry because the need for new treatments and the regulatory requirement for clinical trials are constant.
- Diverse Career Paths: The field has a well-defined career ladder with multiple specialty options. You can start in roles like a Clinical Research Coordinator (CRC) or Clinical Trial Assistant (CTA) and progress into high-level positions such as
- Clinical Research Associate (CRA)
- Clinical Trial Manager (CTM)
- Project Manager
- Regulatory Affairs Specialist
- Clinical Data Manager
- Competitive Salary: With experience and specialization, the salary potential in clinical research, particularly in the industry (CROs and Pharma), is very competitive.
- Global Opportunities: Clinical trials are conducted worldwide, meaning the skills and certifications you acquire are globally transferable, opening doors to international travel and employment.
🚪 Accessibility for Life Science Graduates
Clinical Research is particularly accessible to those with a background in Microbiology, Biotechnology, Biochemistry, Zoology, Pharmacy, etc.
- Relevant Backgrounds: It’s an excellent field for students of pharmacy, life sciences, biotechnology, and medicine.
- Entry-Level Opportunities: Many freshers start in entry-level roles such as a clinical research coordinator, managing the day-to-day operations of a clinical trial at a hospital or site. Responsibilities include patient recruitment, obtaining informed consent, collecting data, and ensuring compliance with the study protocol.
How Has the Industry Worked Out So Far?
The clinical research industry has generally worked out well, experiencing significant growth and undergoing a rapid technological and operational transformation in recent years.
📈 Current Status and Market Growth
The global clinical trials market is a massive and expanding industry:
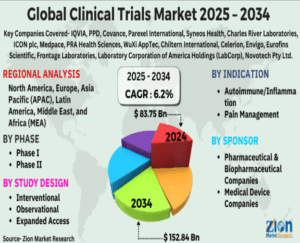
- Market Size: It was estimated to be valued at around $80 – $90 billion in 2024/2025.
- Growth: It is projected to continue growing at a strong Compound Annual Growth Rate (CAGR), potentially reaching over $150 billion by 2033.
- Key Drivers:
- Rising Prevalence of Diseases: The increasing global burden of chronic and rare diseases (especially oncology, which is the largest therapeutic area) fuels the demand for new treatments.
- R&D Investment: Continued high R&D spending by pharmaceutical and biotech companies.
- Outsourcing: Increasing reliance on Contract Research Organizations (CROs) to manage trials, leveraging their expertise and cost advantages.
💡 Major Trends Shaping the Industry
The industry is in a phase of modernization, driven by technology and a focus on the patient:
- Decentralized Clinical Trials (DCTs): This is one of the most significant shifts. DCTs use digital tools, wearables, and telemedicine to allow participants to take part remotely, reducing the burden on patients and expanding trial access and diversity.
- Adoption of AI and Machine Learning (ML): These technologies are being used to:
- Optimize Trial Design: Making studies more efficient.
- Enhance Patient Recruitment: Predicting and identifying suitable participants faster.
- Analyze Data: Processing the massive amounts of data generated, especially from wearables.
- Patient-Centricity: There’s a growing emphasis on designing trials that prioritize the patient experience, including easier protocols, better communication, and greater patient input.
- Focus on Diversity and Inclusion: Regulatory bodies worldwide are pushing for greater diversity in trial populations to ensure new therapies are safe and effective across all demographic groups.

This change has led to the creation of a huge number of job opportunities related to this sector. Clinical research and Pharmacovigilance courses make you a step ahead in standing and getting hired in this industry. The CR market is moderately competitive and consists of several m
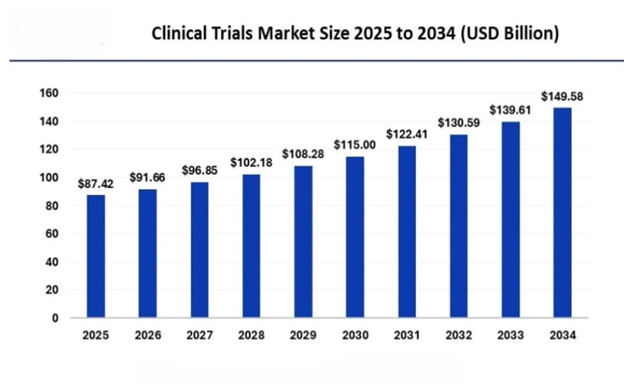
ajor players. Companies like IQVIA, ICON, Fortrea, Syneos, Parexel, and PPD hold a substantial market share in the CR market. The Asia Pacific market accounted for a significant industry share in 2024 and is poised to exceed USD 149.58 billion by 2034, according to a new report published by Global Market Insights, Inc.
Job and career prospects
The growth in CR is fast, which eventually leads to managerial and director roles with good pay packages.
An entry-level job for life science graduates/postgraduates is CRC. CRCs are mainly involved in Day-to-day operations at the clinical site, including patient recruitment and data collection.
From there, career progression in CR will typically grow with exposure, knowledge, and experience. Your role can vary from process expert, subject Matter expert, rt product quality lead, team leader, assistant manager, deputy manager, senior manager, general manager, director, vice president, Service Delivery Manager/lead, and many more. There is continuous growth in the field with an average 30% raise in salary every year. The yearly compensation for a career in CR is very g, good and the starting salary ranges.
Apart from these, your role can also involve a more in-depth technical part if you acquire the additional skills in CR as below. Salary packages reach high with each role or designation up to 50-60 lakhs or more per annum based on experience, tenure, and knowledge and skills.
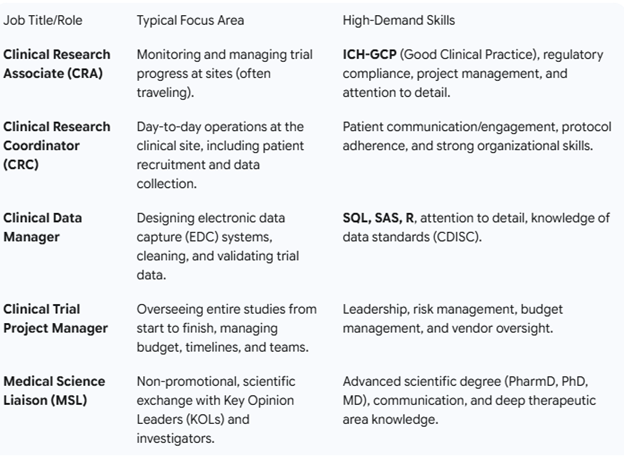
Growth Prospect:
The clinical research field offers stable, high-demand, and rewarding career prospects for those with a background in life sciences, pharmacy, or medicine. The long-term growth prospects for the clinical research industry are exceptionally strong, driven by a convergence of scientific, demographic, and technological factors. This translates into a highly stable and expanding job market.
What Training programs/courses can be taken to become a Clinical Research Professional at Dysmech Clinical Services Pvt Ltd (DCS)
- Post Graduate Diploma in Advanced Clinical Research Management (CRM) (1 year) (9 months theory + practical & 3 months internship)
- Post Graduate Diploma in Clinical Research (CR) and Pharmacovigilance (PV) (1 year) (9 months theory + practical & 3 months internship)
- Post Graduate Diploma in Clinical Research (CR) and Clinical Data Management (CDM) (1 year) (9 months theory + practical & 3 months internship)
- Post Graduate Diploma in Clinical Research (CR) and Regulatory Affairs (RA) (1 year) (9 months theory + practical & 3 months internship)
- Advanced Diploma in Clinical Research Management (CRM) (6 months theory + practical & 1 month internship)
- Diploma in Health Technology Assessment (HTA) (6 months)
- Diploma in Good Clinical Practices (GCP) (6 months)
- Diploma in Advanced Clinical Research (CR) and Pharmacovigilance (PV) (6 months theory + practical & 1 month internship)
- Diploma in Clinical Research (CR) and Clinical Data Management (CDM)
(6 months theory + practical & 1 month internship)
- Diploma in Clinical Research (CR) and Regulatory Affairs (RA) (6 months theory + practical & 1 month internship)
- Certificate course in Clinical Research (CR) (3 Months)
- Certificate course in Pharmacovigilance (PV) (3 Months)
- Certificate course in Clinical Data Management (CDM) (3 Months)
About Dysmech Clinical Services Pvt Ltd (DCS):
DCS is an educational research centre, a professional training and soft skill development organization. Our company provides the best and state-of-the-art facilities and knowledge-sharing platforms, which are an exception in the CR.
DCS offers specialized programs with industry exposure in international standards with the Drug Information Association (DIA)
Our faculty and guest lecturers are from the industry itself, and they are astute in delivering their duties as and when required by the students. Solving the queries of the students and taking them to the right path is what our faculty is masterful in.
The ambience and study environment at @DCS is one of the best compared to the other institutes providing Pharmacovigilance courses in Pune.
The teaching staff at @DCS helps to learn the depth of this subject and course, and helps them get certified, which h internationally acclaimed in collaboration with some of the prestigious partners.s
Drug Information Association, which adds volume to their profile and makes each of our students get a practical exposure to software tools along with theory classes, hi which help them train and become proficient to enter the industry.
Students registered with us @DCS would have an excellent platform and knowledge to land in a good company or enterprise, and can boost their growth opportunities by availing the benefits of our wide network and contacts across companies and industries.
Looking at the growing demand for the courses, DCS has introduced the state-of-the-art curriculum, which will help our students cope with the requirements and pressures of the real job. For the benefit of working professionals and full-time students, the course is conducted every Saturday.
You can very well trust in our honest dealings.
So, when are you joining??
Check out the details and contact us today to be the leader of tomorrow.

